Cytogenetically normal acute myeloid leukemias (CN-AML) represent about 50% of total adult AML. Despite the well-known prognosis role of gene mutations such as NPM1 mutations or FLT3 internal tandem duplication (FLT3-ITD), clinical outcomes remain heterogeneous in this subset of AML. Given the role of genomic instability in leukemogenesis, expression analysis of DNA repair genes might be relevant to sharpen prognosis evaluation in CN-AML.
Publicly available gene expression profile dataset from two independent cohorts of patients with CN-AML were analyzed (GSE12417). A list set of 175 genes involved in six major DNA repair pathways (base excision repair (BER), NER, mismatch repair (MMR), homologous recombination repair (HRR), non-homologous end joining (NHEJ) and FANC pathways) was defined using the REPAIRtoire database (http://repairtoire. genesilico.pl) and review of the literature. We investigated the prognostic value of these 175 genes involved in DNA repair. Among these genes, 23 were associated with a prognostic value, using the MaxStat R function. To further corroborate gene expression data on a functional level, CRISPR or RNAi screening publicly available data were used (Dependency Map data, Broad Institute, www.depmap.org). Among the 19 genes associated with a poor outcome, APEX (BER), RTEL1 (HRR) and COPS6 (NER) were identified as significant essential AML genes (p = 7.9e-05, 3.4e-04 and 2.8e-04 respectively). The prognostic information provided by these 23 genes was summed (sum of the beta coefficients of the Cox model for each prognostic gene, weighted by +1 or -1 according to the patient signal ≥ the probe set MaxStat value) in a DNA repair score to consider connection of DNA repair pathways.
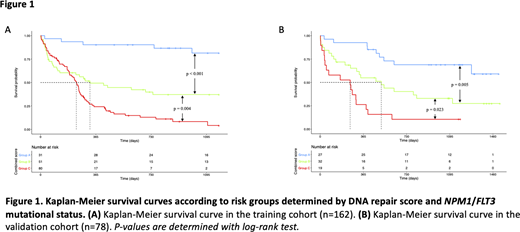
In the CN-AML training cohort (n=162), DNA repair score allowed to define a group of patients (n=87; 53,7%) with poor median overall survival (OS) of 233 days (95% CI: 184-260). These results were confirmed in the validation cohort (n=78) (median OS: 120 days; 95% CI: 36-303). In multivariate Cox analysis, the DNA repair score, NPM1and FLT3-ITD mutational status remained independent prognosis factors in CN-AML. Therefore, we investigated the interest of combining DNA repair score and NPM1/FLT3 mutational status to predict CN-AML outcome. Patients were classified according to prognosis value of DNA repair score (0 point for group I; 1 for group II; 2 for group III), and NPM1/FLT3 mutational status (0 point if NPM1 mutation without FLT3-ITD; 2 points if FLT3-ITD without NPM1 mutation; 1 point in other situations). The sum of the prognostic information was computed for all patients, allowing to separate patients in three new prognostic groups: group A including patients with 0 or 1 point, group B for patients with 2 points and group C for patients with 3 or 4 points. Combining these parameters allowed the identification of three risk groups with different clinical outcomes in both training and validation cohorts (Figure 1). In the training cohort, median OS was not reached (95% CI: NR-NR), 326 days (95% CI: 127-NR) and 236 days (95% CI: 190-263) respectively for patients in groups A, B and C. One-year OS was 90.3% (95% CI: 80.5-100) in group A, 49.3% (95% CI: 37.1-65.7) in group B, and 24.2% (95% CI: 16.2-36.2) in group C.These results were confirmed in the validation cohort where median OS was not reached (95% CI: 1278-NR), 516 days (95% CI: 308-NR) and 253 days (95% CI: 52-403) for patients respectively in groups A, B and C. One-year OS was 92.6% (95% CI: 83.2-100) in group A, 54.9% (95% CI: 39.8-75.7) in group B, and 26.5% (95% CI: 12.4-55.8) in group C. OS was statistically different between groups A, B and C in both training and validation cohorts. Combined with NPM1 and FLT3 mutational status, our GE-based DNA repair score might be used as a biomarker to predict outcomes for patients with CN-AML. DNA repair score has the potential to identify CN-AML patients whose tumor cells are dependent on specific DNA repair pathways to design new therapeutic avenues.
Cartron:Celgene: Consultancy, Honoraria; F. Hoffmann-La Roche: Consultancy, Honoraria; Sanofi: Honoraria; Gilead: Honoraria; Jansen: Honoraria; Abbvie: Honoraria. Moreaux:Diag2Tec: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal